The US Department of Health and Human Services (HHS) is planning to require placebo-controlled trials for all new vaccines prior to licensing. Because the vaccines for evolving viruses, such as influenza and COVID are updated each season, they could be considered new, so would need to undergo the placebo trial testing. This would not only add to the costs of the vaccine but would also significantly increase the time to deployment, potentially limiting availability, and raise ethical concerns.
It also has become a contentious issue because HHS has not provided further information on the plan, and the base version of most vaccines have undergone a placebo trial during their Phase 3 approval process. Experts see requiring placebo tests of updated versions as being unethical because it would be “withhold(ing) a shot known to be safe from a particular group, and because the shot is only being tweaked in a minor way.” Additionally, because of the high cost involved, “drug makers could ultimately decide to forgo making the newer, more effective versions of the vaccine altogether.”
But controversy on vaccine trials is not new, particularly the use of unvaccinated controls (with or without placebo) when an efficacious vaccine already exists. While randomized, placebo-controlled trials are the “gold standard” for evaluating vaccine safety and efficacy, the WHO advises using placebos in limited specific circumstances. Evaluating an existing vaccine’s local safety and efficacy might justify placebo use, but only when the information cannot be obtained through an active-controlled trial design, the risks of delaying or withholding an effective vaccine are properly mitigated, the social and public health value of the research justifies the risks of using a placebo control, and the research addresses specific local health needs.
Additionally, according to the research ethics guidance published by the Council for the Organisation of Medical Sciences (CIOMS), in collaboration with WHO, as a general rule, research participants in the control group of a trial must “receive an established effective intervention” unless there are compelling scientific reasons for doing so or delaying or withholding the intervention would cause only minimal risk.
In December 2020, as COVID-19 vaccines were first becoming available for human use but remained limited to priority populations, a WHO ad hoc consultation committee recommended that while vaccine supplies remained scarce and available vaccines were still investigational, researchers should continue using directly randomized comparisons against placebo to gather critical data. They concluded it was ethically appropriate to maintain blinded follow-up of placebo recipients in both existing and new trials, allowing scientists to collect reliable information on long-term safety and efficacy that would otherwise be difficult to obtain through observational studies. This approach would help address significant knowledge gaps regarding duration of protection, potential late-emerging side effects, and effectiveness across different populations – information crucial for building public confidence in vaccination efforts.
As the debate is not new, it is unlikely to be resolved soon, but we will be keeping close watch on the HHS developments and specifics on the plans for and specific vaccine application of the stated placebo-trial requirements. Ultimately, public health is best served by a balanced framework that ensures vaccine safety while enabling timely adaptation to emerging viral variants; a balance that rigid placebo requirements for all vaccine modifications may disrupt, potentially undermining both innovation and public confidence in vaccination programs.
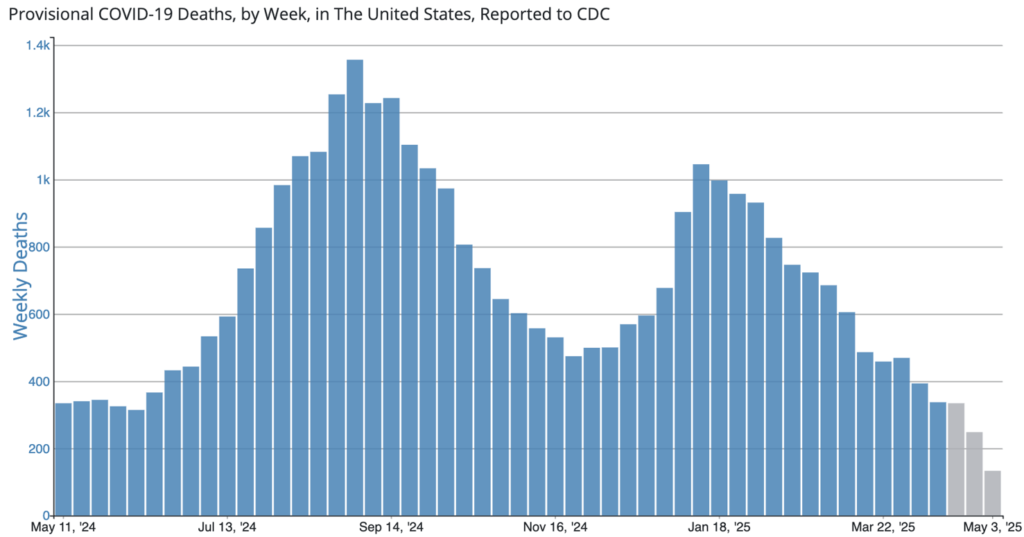
COVID Risk Matrix:
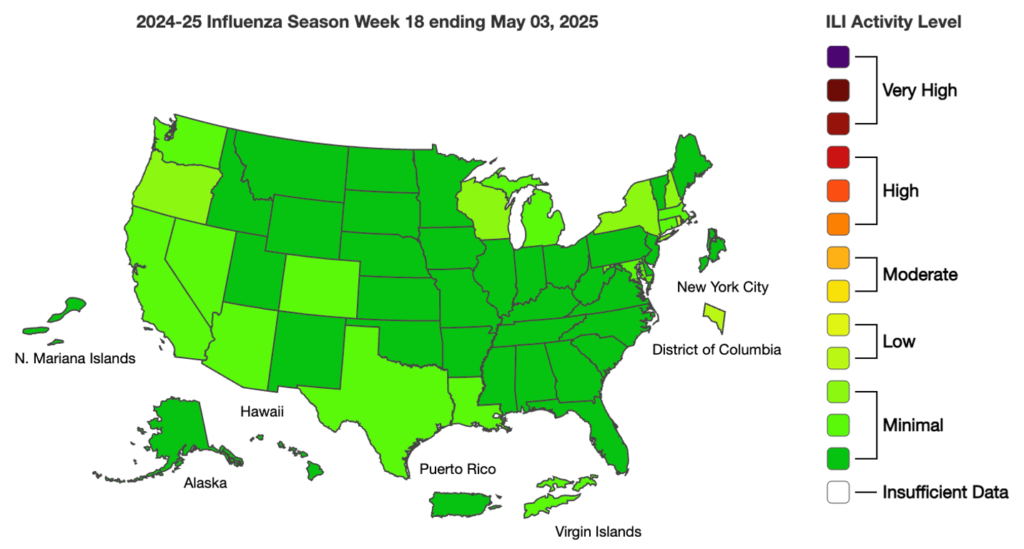
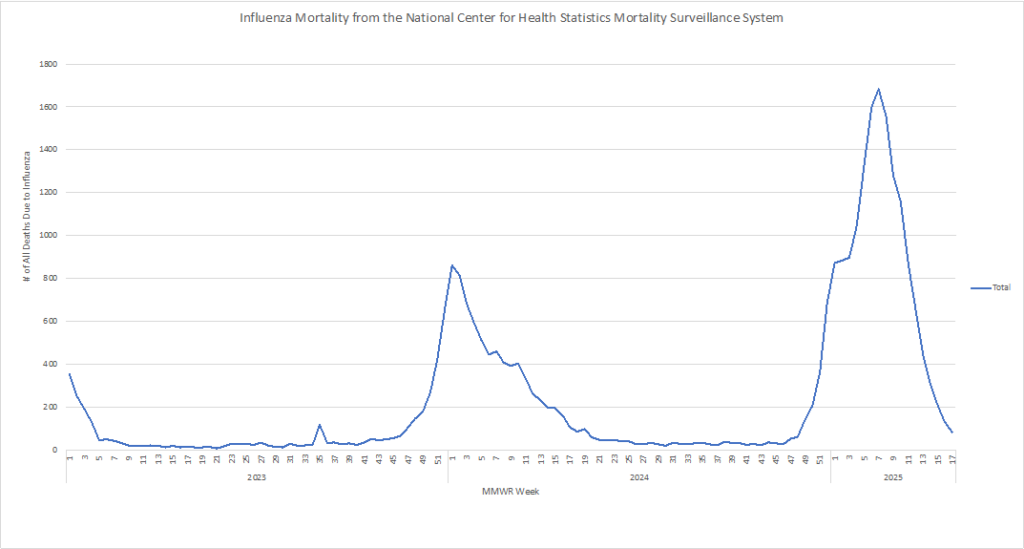
Influenza:
Infectious Disease News:
- A new study led by CUNY SPH researchers warns that public indifference and misinformation about bird flu (HPAI) could undermine efforts to prevent a larger outbreak. Many Americans lack basic food safety knowledge, are reluctant to change diets or accept vaccination, and show partisan and geographic divides in response. Experts stress the need for targeted communication, especially in rural and agricultural communities, to curb the risk of a public health crisis.
- As of April 26, 2025, Canada has reported 1,506 measles cases (1,299 confirmed and 207 probable). Ontario has reported the majority of the cases, but cases are throughout 7 provinces.
- The Trump administration has disbanded the Healthcare Infection Control Practices Advisory Committee (HICPAC), a key CDC advisory group that has shaped infection prevention guidance in U.S. healthcare settings for over three decades. The termination, part of a broader effort to reduce federal advisory bodies, was communicated to members via letter last week, just ahead of a scheduled May meeting. HICPAC’s evidence-based recommendations have informed national standards on hand hygiene, isolation protocols, PPE use, and infection control in hospitals and clinics.
- A new JAMA study reports that Moderna’s combined COVID-19 and flu vaccine, mRNA-1083, produced stronger immune responses than standard separate shots in adults 50 and older. The vaccine was safe, with no serious side effects, though mild reactions were more common. Researchers say the mRNA platform could simplify seasonal vaccination and improve protection.





