The FDA has approved new, updated COVID-19 vaccines, which have been formulated to more closely target current variants and provide better protection against severe consequences. The approval and authorization was made on Monday. The actions approve Moderna (Spikevax) and Pfizer (Comirnaty) 2023-24 updated mRNA vaccines which include a monovalent (single) component that corresponds to the Omicron variant XBB.1.5. The vaccines are approved for individuals 12 years of age and older and are authorized under emergency use for individuals 6 months through 11 years of age.
In agreement, the CDC’s Prevention’s Advisory Committee on Immunization Practices (ACIP) met on Tuesday and voted 13 to 1 to recommend the “2023-2024 (monovalent, XBB-containing) COVID-19 vaccines as authorized under Emergency Use Authorization (EUA) or approved by Biologics License Application (BLA) in person >6 months of age.” Both the FDA actions and ACIP recommendations mean that the previous bivalent Moderna and Pfizer-BioNTech COVID-19 vaccines are no longer authorized for use in the US.
Specifically:
- Individuals 5 years of age and older regardless of previous vaccination are eligible to receive a single dose of an updated mRNA COVID-19 vaccine at least 2 months since the last dose of any COVID-19 vaccine.
- Individuals 6 months through 4 years of age
- If previously vaccinated, can receive one or two doses of an updated vaccine.
- If previously unvaccinated, can receive three doses (Pfizer) or two doses (Moderna).
- Individuals who receive an updated mRNA COVID-19 vaccine may experience similar side effects as those reported with previous vaccines.
- The updated vaccines are expected to provide good protection against current COVID-19 variants with annual updates, as is done for the seasonal influenza vaccine.
The updated mRNA vaccines are manufactured using a similar process as previous formulations with similar efficacy against the current COVID-19 strains as were attained with prior versions of the vaccines against prior variants. Additionally, FDA’s benefit-risk assessment demonstrated that the benefits of these vaccines for individuals 6 months of age and older outweigh their risks.
Manufacturers have announced that the updated vaccines will be ready this fall, and the FDA expects the updated vaccines to be available in the near future. Those who are eligible are encouraged to consider getting vaccinated.
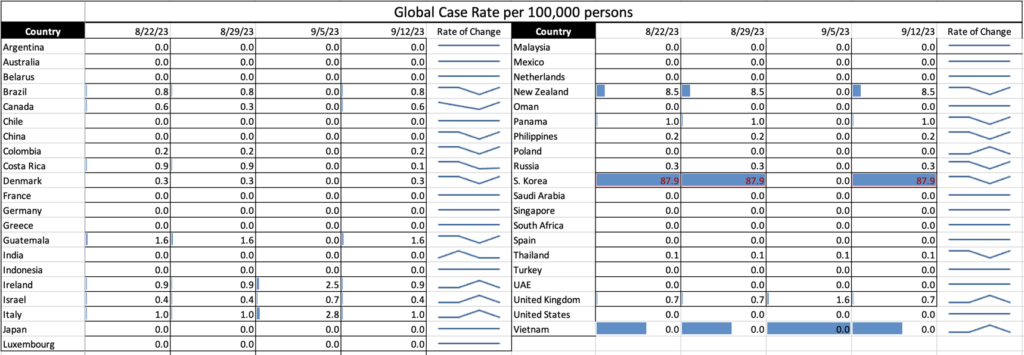
COVID Risk Matrix:
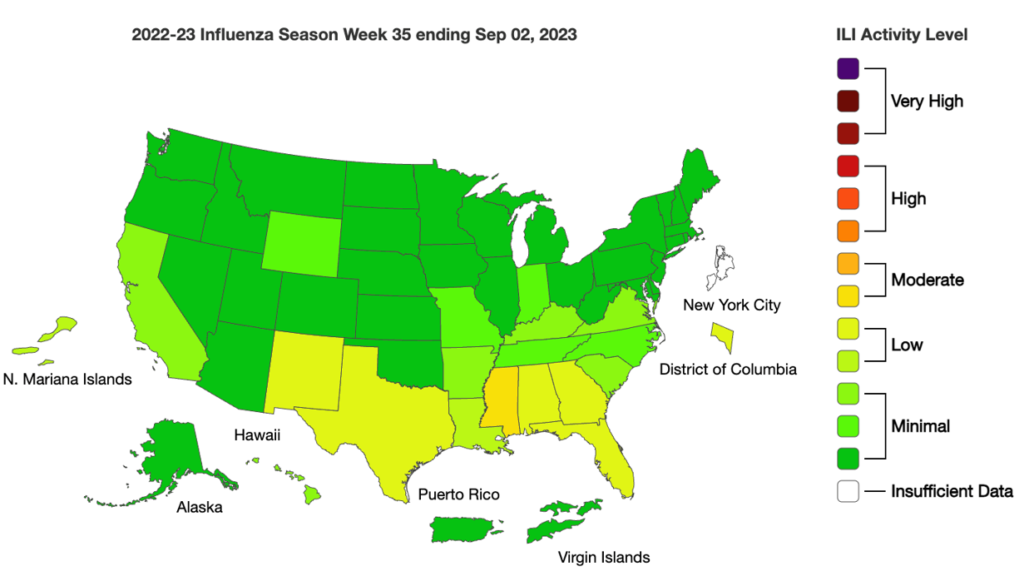
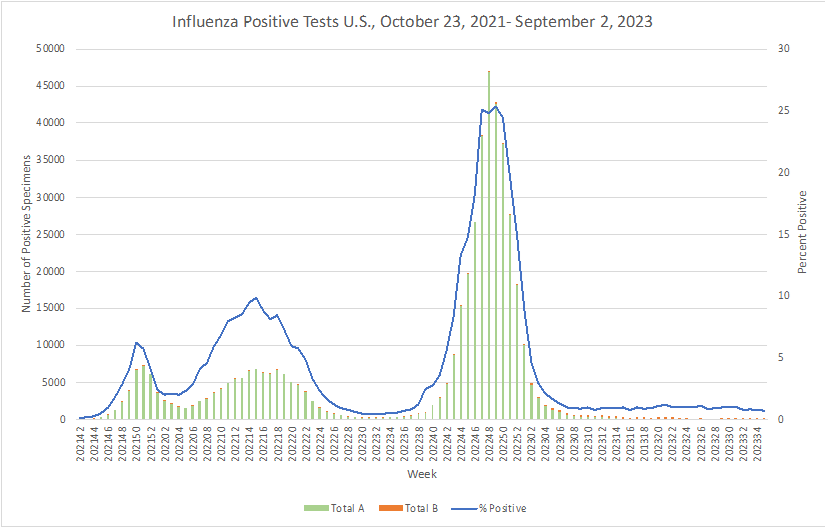
Influenza:
- Eleven Calgary daycares were impacted due to Shiga toxin-producing E.coli cases running through children. The case count as of September 12, was at 264. 27 people were hospitalized and 22 have been confirmed to have hemolytic uremic syndrome (HUS). One kitchen is shared between all locations, and that is likely where the outbreak rooted. Cockroaches were a previous violation of the kitchen, along with a “significant evidence of pest infestation.” Additionally, during inspection, there were instances where food handling was not up to par.
- The FDA and ACIP have recommended the 2023-24 monovalent COVID vaccine from Moderna and Pfizer for emergency use. The current bivalent COVID vaccine which is available should no longer be used. Additionally, Health Canada has approved the use of the updated Moderna COVID vaccine for individuals 6 months and older in Canada.
- Insect-transmitted diseases such as Lyme disease and West Nile, among others are on the rise. Health officials believe there is a significant under-diagnosis and under-reporting of less severe cases of vector-borne diseases. Many researchers believe that climate change is linked to the rise of many insect-transmitted conditions. Scientists are currently warning individuals that these types of infections are on the rise across North America and to seek medical attention if you believe you have contracted an infection.
- China health authorities are recommending flu vaccines for everyone older than 6 months old by November.
- Measles cases are up this year in England, India and Romania, when compared to the years of the pandemic. It is suspected that restrictions put in place to help stem the spread of COVID may have had an effect on measles transmission as well.
- Since May in Denmark, an increasing number of cases of whooping cough have been registered, and during the summer the number has been steadily increasing. The increase has been seen throughout the country with the number of detected cases four times higher than normal. A total of 1,229 cases of whooping cough have now been detected in 2023. There is a marked increase from 12 detected cases in January to 439 cases in August. There is a particularly high incidence among young children aged 0 years, among older children aged 9 to 19 years and among adults aged 40 to 50 years. It is well known that whooping cough occurs in epidemics three to five years apart and that the vaccine may not offer lifelong protection.