Respiratory viruses tend to spread at heightened rates in the fall and winter, peaking from December to February. Thus, fall vaccination against the viruses is recommended for influenza, COVID, and respiratory syncytial virus (RSV). Following are some specific recommendations for each:
RSV. In May, FDA approved Arexvy as the first RSV vaccine for use in the United States. It is approved for use in those 60 years and older for the prevention of lower respiratory tract disease caused by RSV. It is recommended that those who are eligible get the vaccine now, particularly those with underlying health conditions, such as heart or lung disease or weakened immune systems, who are at high risk for severe illness.
COVID. With the updated mRNA vaccines now FDA-approved and available, it is recommended that you schedule your vaccine now with your health-care provider or local pharmacy (some of which accept walk-ins). In last week’s article, we discussed the new vaccines and eligibility for them.
Influenza. Each year the flu vaccine is updated to be most effective against circulating strains. While it is important that all who are eligible get vaccinated, CDC recommends that vaccination be scheduled by the end of October. Because protection can wane over time, it is best for adults to not get vaccinated too early unless public health indicators in the local area demonstrate increasing cases. But everyone, aged 6 months and older, should be vaccinated every season, particularly those who are at higher risk – even if you have already had the flu this season, as it will protect against other strains. For babies too young to be eligible for a vaccine, the best protection to is ensure those around them are vaccinated. Additionally:
- There are several flu shots approved for use in those 6 months old and older.
- Three vaccines are recommended for those 65 years and older, with two of those approved only for this age group.
- Flu shots are recommended for those who are pregnant and those with certain chronic health conditions.
- A nasal spray flu vaccine is approved for use in those 2 to 49 years of age. It is not approved for those who are pregnant or have certain medical conditions.
- Stay connected with local public health communications to be advised of flu illness rates in the area.
While there are varying recommendations for vaccination for each of the respiratory viruses, the most important thing is that all who are eligible for each be vaccinated every year.
COVID Risk Matrix:
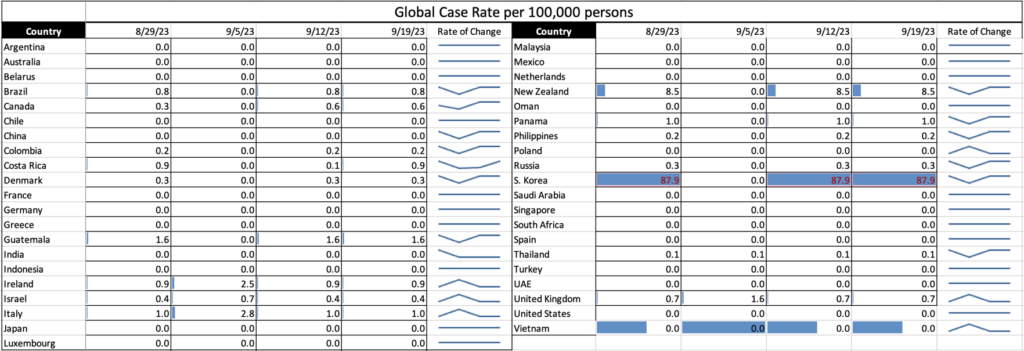
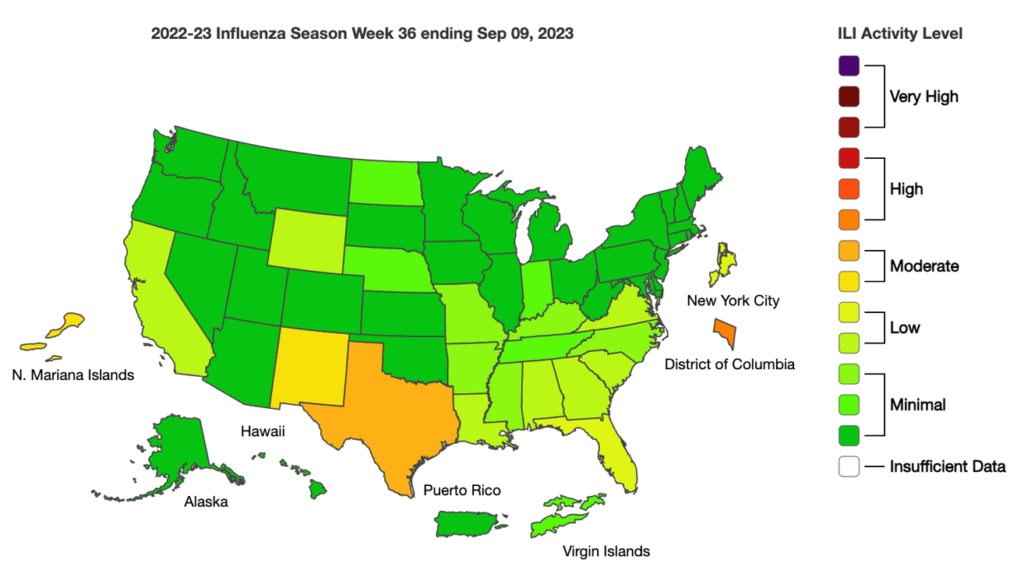
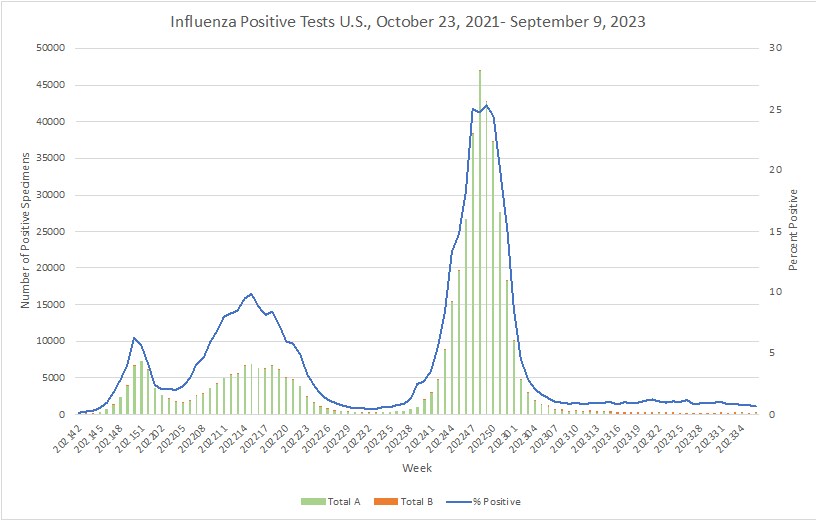
Influenza:
- There is a current outbreak of Nipah virus in India, specifically in Kerala. There have been two deaths reported and six confirmed cases so far. Health officials have closed schools and offices in the area to prevent the spread. There are currently no specific treatments for the virus, and it carries a high fatality rate. Nipah is a zoonotic disease that was first discovered in 1999 in pigs and people. Common symptoms include fever, headache, cough, sore throat, difficulty breathing and vomiting, and the virus can lead to more severe symptoms.
- There are now 348 confirmed individuals with Shiga toxin-producing E. coli in the Calgary daycare outbreak. Nine patients are currently receiving treatment for HHS in the hospital while three patients are on dialysis. Other schools and daycares in the area have been closed or partially closed due to what is believed to be a secondary spread.
- The CDC has reported US COVID-19 hospital cases are up slightly. Hospitalizations are up 7.7% and deaths are up 4.5% from the most recent reporting week. Additionally, WHO reported that global cases have increased by 38% but deaths have decreased by over 50% over the past 28 days.
- Penn State was awarded a grant from the USDA for $4.5 million to test for SARS-CoV-2 in 58 wildlife species, with the goal of tracking potential human spillback. The researchers plan to sample the same animals in the same region multiple times to determine whether and how transmission is occurring.