TAG continues to share insightful information about public health-related topics to dig deeper into the issues that matter so you can be better educated and empowered to react appropriately. This week’s article, which is the third in the series, continues the focus on color additives.
The FDA regulatory framework for the use, labeling, and safety of color additives which was defined by Congressional legislation through the Color Additive Amendments of 1960 to the Federal Food, Drug and Cosmetic Act (FFDCA) of 1938. Of the approximately 30 food colors listed at that time, 20 were exempt, such as: extracts from annatto and grape skin; carrot oil; paprika, saffron, and other spices; and fruit and vegetable juices. The remaining synthetic colors, sometimes called “artificial,” were non-exempt, required certification, and included the FD&C colors. Subsequent FDA regulations establishing strict requirements for the use of approved exempt food color additives are found in 21 CFR 73 Subpart A and non-exempt (certified) food color additives are found in 21 CFR 74 Subpart A. A summary of all the color additives approved for food (and other applications like drugs, cosmetics, etc.) lists the specific regulation, the year it was approved, and the uses and restrictions.
Certification of synthetic color additives is not the same as FDA approval. Certification means an FDA chemist has tested a batch sample and found it meets the required standards for composition and purity set by regulations for that color additive. FDA scientists evaluate the batch for physical appearance and chemically analyze it for purity (total color content), moisture, residual salts, unreacted intermediates, colored impurities other than the main color (subsidiary colors), any other specified impurities, and the heavy metals lead, arsenic, and mercury. Use of an approved synthetic color additive that has not been certified makes the color adulterated under the law and subject to FDA enforcement and acting against food products that use that color.
The FDA has published guidance for industry on color additive petitions that details the agency’s recommendations for the submission of the required chemical and technological data. For the most part, the requirements mirror those found for food additive petitions to ensure safety of the substance, including any impurities. Regardless of source, whether synthetic or from “natural” sources, every food color additive must go through the same strict premarket review process involving the submission of a color additive petition. All told, the process to gain certification and, therefore, market access takes a minimum of five years and often upwards of 15 years, from R&D to regulatory approval. FDA decides which color additives are non-exempt and require certification based on whether they might contain impurities that pose a health concern.
Although it is commonly believed that the United States approves more synthetic color additives for food use than other countries—including some that are prohibited elsewhere—the reality is quite the opposite. Including the currently approved use of Erythrosine (FD&C Red No. 3), which is soon to be delisted, there are nine synthetic food color additives currently approved in the U.S. compared to 10 in Canada, 14 in Australia/New Zealand, and 15 in Europe.
FDA continuously monitors information relating to the safety of all color additives, including considering work from the Joint FAO/WHO Expert Committee on Food Additives (JECFA), an international health authority. Food businesses that use colors must remain well informed about the safety of these substances, by accessing the most current technical information from among the many credible sources. Typically, regulatory personnel at food companies take on this responsibility to ensure they are in line with the most credible science.
The next and last in the series of four articles on additives will cover developments for food and color additives, including FDA’s recent announcement on the granting of three new food colors from natural sources.
COVID Risk Matrix:
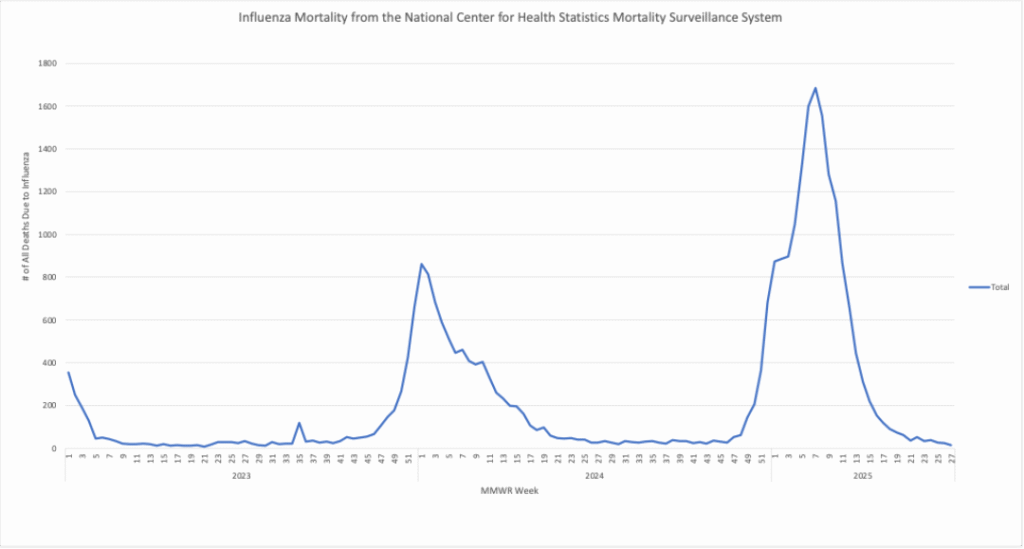
Influenza:
Public Health News:
- As of July 14, 2025, the Australian Capital Territory, Queensland, and South Australia are reporting higher flu case numbers compared to previous years, although the numbers are below 2024 levels. Seasonal influenza trends in Australia, which typically peak in winter months, may provide early insight into the upcoming Northern Hemisphere flu season.
- The CDC has published a reminder that adults need certain vaccines too! This link takes you to a handy chart that shows recommended vaccines at different ages.
- In the Kerala state in India, the son of a man who died recently from a Nipah virus infection has also become infected. This zoonotic virus, which is spread by fruit bats and by consuming palm sap or fruits contaminated with bat excreta, can be transmitted from human-to-human contact as well. Health officials are conducting contact tracing. The Nipah virus causes symptoms ranging from respiratory infection to encephalitis, with a case fatality rate as high as 75%.
- The European CDC warns that the risk of Vibrio infections increases during warm summer months and sea surface temperatures. Transmission can occur through consumption of raw or undercooked shellfish, which causes gastrointestinal symptoms, or through cuts or wounds on the skin.
- An epidemiological study of the incidence of African Swine Fever (ASF) in the EU revealed a decrease in outbreaks in domestic pigs by 83%, led by fewer reported outbreaks in Croatia and Romania. Most outbreaks occurred on smaller farms with fewer than 100 pigs. ASF also occurs in wild boars, but those outbreak numbers have remained stable.





