This is the last of four TAG newsletter articles that examined safety as well as FDA regulatory oversight of food and color additives. The previous articles were: FDA Approval of Food and Color Additives; Safety of Color Additives in Food; Regulatory Oversight of Food Color Additives. As the understanding of food science continues to evolve, so must the evaluation of the safety and regulatory processes for approving food and color additives. The FDA keeps abreast of developments necessary for assuring the safety of the food supply as evidenced by the recent announcement on May 9, 2025, for the approval of three food color additives. These color additives are: (1) Galdieria extract blue, (2) Butterfly pea flower extract, and (3) calcium phosphate. The FDA should be commended for their timely approval of these color additive petitions to meet consumer needs to expand the portfolio of colors additives beyond the currently approved ones. With these approvals, any manufacturer can use these colors for the approved food applications.
Along with these new color approvals is an initiative by the FDA to phase out the use of synthetic, non-exempt food color additives by working with the industry to eliminate six colors (FD&C Green No. 3, FD&C Red No. 40, FD&C Yellow No. 5, FD&C Yellow No. 6, FD&C Blue No. 1, and FD&C Blue No. 2) from the food supply by the end of 2027. In addition, the FDA plans to revoke the authorization of two synthetic food colors – Citrus Red No. 6 and Orange B – that are rarely if ever used, in the next few months. As already mentioned in the TAG newsletter of January 21, 2025, the agency is requesting companies remove the use of FD&C Red No. 3 sooner than the original 2027-2028 deadline.
Instead of a lengthy regulatory process to ban certain color and food additives, it is encouraging to see the FDA working with industry to voluntarily remove additives that consumers view as health risks. This approach in many cases preserves science and allows for a future change in consumer perception for a renewed use of an additive previously consider “unhealthy”.
Not since the implementation of Prop. 65 in California in 1986 has the food industry faced a statutory requirement to warn consumers about the presence of certain food and color additives in products. The states of Texas and Louisiana have enacted similar but distinct label requirements warning consumers about several dozen food and color additives. In Texas, Senate Bill 25 signed into law on June 22, 2025 effective September 1, 2025, will require food labels developed or copyrighted after January 1, 2027 containing one or more of 44 ingredients bear this warning statement “WARNING: This product contains an ingredient that is not recommended for human consumption by the appropriate authority in Australia, Canada, the European Union, or the United Kingdom.” Similarly, the Louisiana’s Senate Bill 14 signed into law on June 27, 2025 and effective January 1, 2028, requires products containing one or more of 44 food and color additives to include a QR code that directs them a company maintained website with following warning statement “NOTICE: This product contains [insert ingredient here]. For more information about this ingredient, including FDA approvals, click HERE.” While there is significant overlap in the list of additives that fall under each of these state laws, they do not perfectly line up. Importantly, there are certain exemptions for the labeling requirement, such as when a food is labeled, prepared, and sold within a retail food establishment; is regulated by the USDA Food Safety and Inspection Service (FSIS); is used in a drug or dietary supplement; or is used in certain pesticide applications.
One of the more recent developments regarding the introduction of new and innovative food additives, specifically by food manufacturers to assess and deem an ingredient as GRAS, is an initiative by the HHS Secretary Robert F. Kennedy Jr.. This directs the acting FDA commissioner to explore rulemaking to revise its GRAS rule and related guidance to change the self-determined GRAS pathway (i.e., requiring mandatory GRAS notification). The impact to industry of changing the GRAS process is profound as GRAS notice reviews would likely be subject to even longer timelines for review. The FDA currently aims to respond to a GRAS notice within 180 days of filing, although this timeline is often extended, and can be a year or longer to receive a final response from FDA. At a time when the FDA has been subject to resource restrictions, it is unclear how the FDA would be able to prioritize the review of GRAS notices without significant staffing and resource changes.
FDA’s legislative mandate is to provide regulatory oversight to help ensure the food safety of chemicals such as food and color additives. Historically, FDA has been provided with insufficient resources to fully oversee a vast, complicated and global food supply, but has nevertheless managed for the most part to fulfill its mission. Given the ever-increasing intricacy of the food supply and faced with resource restrictions imposed by the current federal administration, the agency will need to continue to utilize innovative computer technologies such as AI and remote inspections, novel approaches such as data sharing, and voluntary industry collaborations versus regulations, in order to continue to play an important role in ensuring that FDA-regulated foods remain safe, abundant, and varied.
The Acheson Group is following these developments and is ready to support clients in navigating this ever-increasing complexity in our regulatory system.
COVID Risk Matrix:
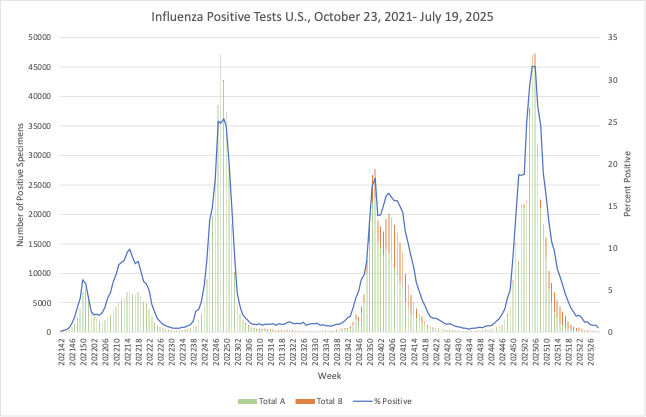
Influenza positive tests throughout the US continue on a downward trend:
Public Health News:
- Researchers at the University of Pittsburgh have estimated via modeling that the burden of influenza in 2022-23, averted by vaccination was as high as 41.5% for seasonal influenza when modeled with a vaccine effectiveness of 40%. They also showed that there was a benefit to unvaccinated people by decreasing the overall level of circulating virus.
- In Sao Paulo, Brazil, more than 900 cases of Hepatitis A have been reported in 2025 to date. This represents a 90% increase over previous years. A source hasn’t been determined, but contaminated food or water is suspected.
- Almost 2000 cases of mosquito-borne chikungunya virus have been reported in southern China; all cases were mild. Mosquito control efforts have been enhanced, including requests to the public to remove standing water that may promote mosquito proliferation. This week, the WHO warned of risks of additional chikungunya virus cases.
- An outbreak of Legionella has been reported in southeast London, Ontario with at least 64 cases and 2 deaths reported. Public health officials are continuing to investigate to determine the possible source, which remains unknown currently.
- A study was recently published focusing on the lack of severe outcomes in humans infected with H5N1, despite about a fatality rate of about 50% in animals. This work using an animal model, concludes that the immunity people have developed to the virus that caused the most 2009 flu pandemic, caused by the H1N1 virus, may have induced some cross-protection that may be making it harder for H5N1 to infect people, and limiting the severity of the ensuing disease if such infections occur. However, while this is a promising finding, further research is needed. The fatality rate in cases of H5N1 in Cambodia is 59%.
- Novavax is proceeding with development of a human H5N1 vaccine. They have announced that preclinical studies in non-human primates demonstrated robust immune responses, so their work continues.
- July 28, 2025, is World Hepatitis Day with a goal of raising awareness of this significant public health challenge and continually working towards preventive efforts.





