With the FDA vaccine advisory group recommending that a monovalent JN.1 lineage vaccine continue to be used in COVID vaccines for the upcoming US respiratory virus season, limits are also being placed on approvals for new vaccines.
As detailed in the FDA approval letter for the new Novavax COVID-19 vaccine (Adjuvanted), the vaccine is approved for individuals 65 and older, and persons 12 through 64 years who have at least one underlying condition that puts them at high risk for severe outcomes from COVID-19.
Additionally, FDA is narrowing its approval for other updated coronavirus vaccines, no longer broadly approving them for the general public. Rather, approval will be limited to use in adults aged 65 and older and those over 6 months, who have health conditions that put them at high risk for severe disease, have conditions such as asthma, diabetes, cancer and obesity; or are pregnant.
In an NEJM article, co-authors FDA Commissioner Dr. Martin Makary and CBER Director Vinay Prasad, M.D., explain the new COVID-19 vaccination regulatory framework as based on immunogenicity, anticipating that the benefits outweigh the risks for adults over the age of 65 and those over 6 months with high-risk conditions. For healthy persons between 6 months and 64 years, however, the FDA “anticipates the need for randomized, controlled trial data evaluating clinical outcomes before Biologics License Applications can be granted.”
Because of these parameters, prescribing the vaccines for healthy people under 65 would be considered off-label use, making it less likely that insurers would broadly cover the shots.
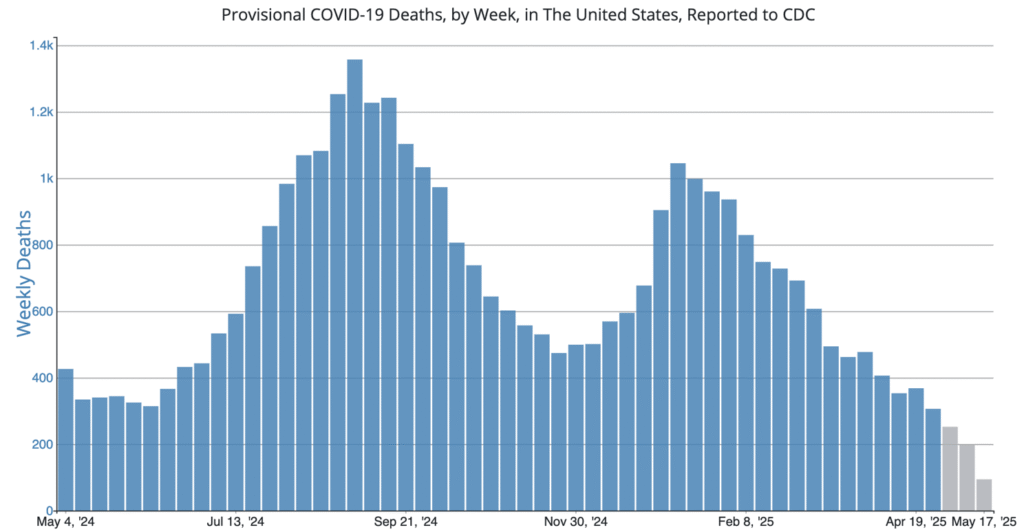
COVID Risk Matrix:
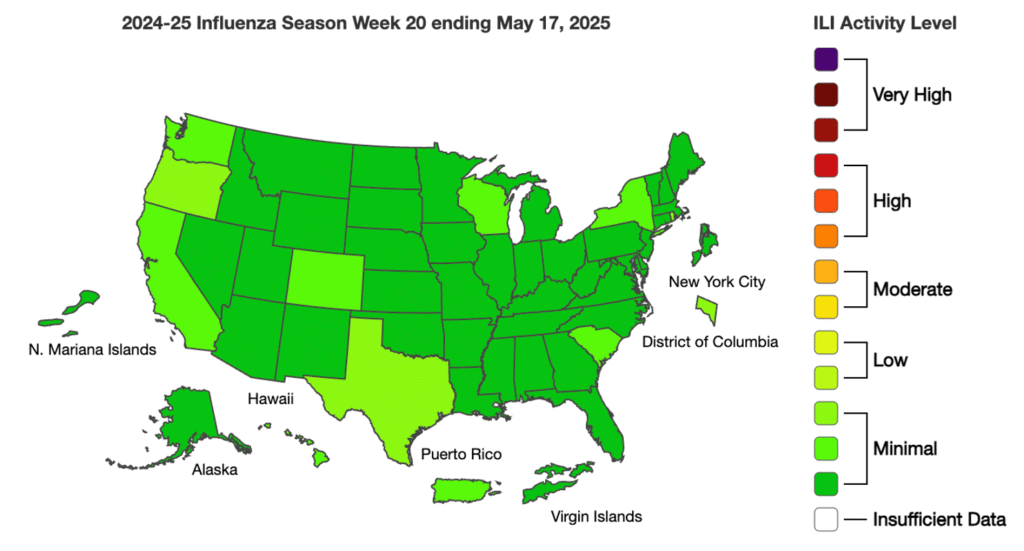
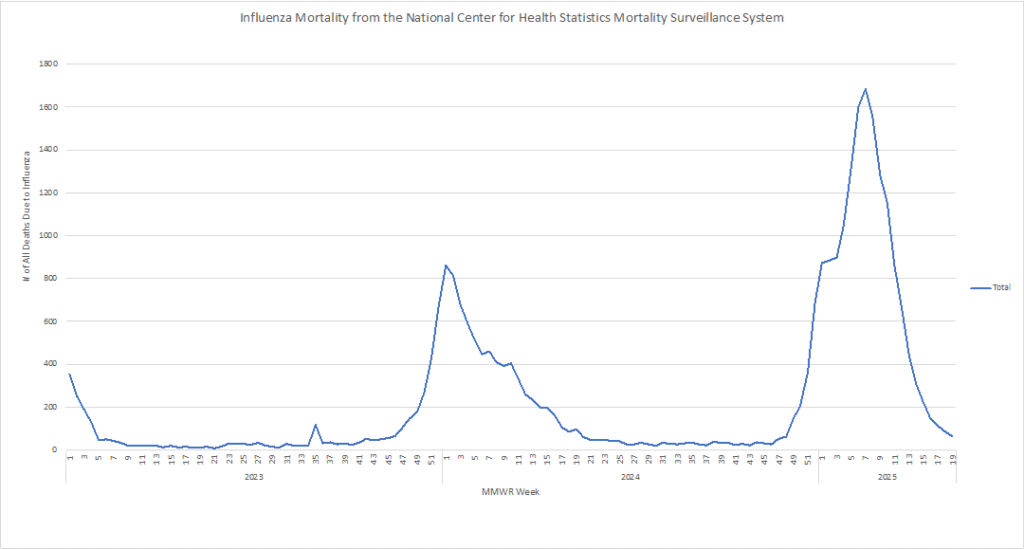
Influenza:
Infectious Disease News:
- Measles update in North America:
- The US has confirmed 1,046 cases have been reported as of May 23. Cases appear to be slowing down.
- As of May 26, Canada has reported a total of 2,515 cases of measles in 2025 (2,197 confirmed and 318 probable).
- The FDA recently advised the manufacturers of the COVID-19 vaccines for those being provided in the fall. The strains should be based on JN.1 lineage strains and preferably the LP.8.1 strain (currently accounts for about 70% of COVID cases in the US).
- The “Make Our Children Healthy Again” report, released by the Make America Healthy Again Commission led by HHS Secretary Robert F. Kennedy Jr., raises concerns about chronic illnesses in children, including questioning the safety of childhood vaccines. The report suggests investigating potential links between vaccines and conditions like autism. It also addresses issues like ultra-processed foods, environmental chemicals, and overmedicalization. Many public health experts and medical organizations have strongly pushed back, reaffirming that vaccines are safe, effective, and vital to preventing serious disease and protecting public health.
- Moderna has withdrawn its licensing application for its combination flu-COVID-19 mRNA vaccine, mRNA-1083, in order to include more efficacy data before resubmitting. This decision follows new FDA guidance limiting COVID-19 booster recommendations to adults 65 and older or those at higher risk. The combo vaccine was originally intended for adults aged 50 and up. Moderna expects to share interim results from its flu vaccine trial, mRNA-1010, later this summer.