Moderna is in Phase 3 of its clinical trial for mRNA-1083, a combined COVID and influenza vaccine. The President of Moderna, Stephen Hoge, announced that the shot will hopefully be available by the fall of 2025, just in time for respiratory disease season that year. The approval of such combination vaccines could advance public health by providing a more convenient option for protection from seasonal illness and respiratory viruses.
Moderna’s mRNA-1083 vaccine combines mRNA-1010, a seasonal flu vaccine candidate, with mRNA-1283, a COVID vaccine candidate. Traditional vaccines work by presenting the body with a harmless amount of virus to trigger an immune response and the production of antibodies. When there is future infection from the same virus, the body recognizes the infectant and fights it more effectively. Rather than using a virus, mRNA vaccines introduce a piece of messenger RNA into the body that causes cells to produce viral protein, against which antibodies form that can protect the body in case of future exposure to that virus. The mRNA does not alter DNA and is broken down naturally after creation of the viral protein.
Clinical trials for new drugs are done in phases, which makes for a long process of production, safety and efficacy testing, and regulatory approval before being marketed to the public. Phase 1 typically tests and monitors the safety and side effects of a new drug on a group of 20 to 100 volunteers. This takes several months to complete, with about 70% of drugs moving on to the next stage of testing. Phase 2 tests efficacy and dosage of the drug on a group of 100 to 300 volunteers, typically utilizing double-blinding and a placebo group. This can take from several months to two years, and only 33% of drugs move on to the next phase. Before being approved by the FDA and released to pharmacies, the drug must successfully complete Phase 3: testing on several hundred to several thousand patients to ensure the drug’s long-term efficacy and safety. This can take one to four years.
In Phase 3 of mRNA-1083, the vaccine is being tested on 2 cohort groups of 4,000 patients each in a randomized, observer-blind control study. In one cohort, individuals 65+ years of age received either mRNA-1083 or co-administered Fluzone HD, an enhanced influenza vaccine, with Spikevax, Moderna’s current COVID-19 vaccine. In the other cohort, individuals 50-64 years old received either mRNA-1083 or co-administered Fluarix, an influenza vaccine, with Spikevax. In both age groups, a single dose of mRNA-1083 was superior in immune responses against three flu strains and SARS-CoV-2. The mRNA technology therefore proved effective, generating greater immune responses than traditional influenza vaccines or the Spikevax shots did separately.
The safety of the mRNA-1083 combination vaccine has also been investigated. Patients have thus far only experienced side effects and reactions of low severity, the most common being injection site pain, fatigue, muscle aches, and headache. Moderna expects to present its Phase 3 data for this clinical trial at an upcoming medical conference followed by publication. Engagement with regulators is also expected to occur soon.
Combining COVID-19 and influenza vaccines into a single dose may improve public health by enhancing compliance and convenience, as it reduces the number of healthcare visits needed. This combined approach is likely to increase people’s willingness to get vaccinated, as it simplifies the process and reduces the time and effort required, leading to higher overall vaccination rates and better protection for the community. This streamlining could also benefit healthcare resources by consolidating vaccination efforts and minimizing logistical challenges.
COVID Risk Matrix:
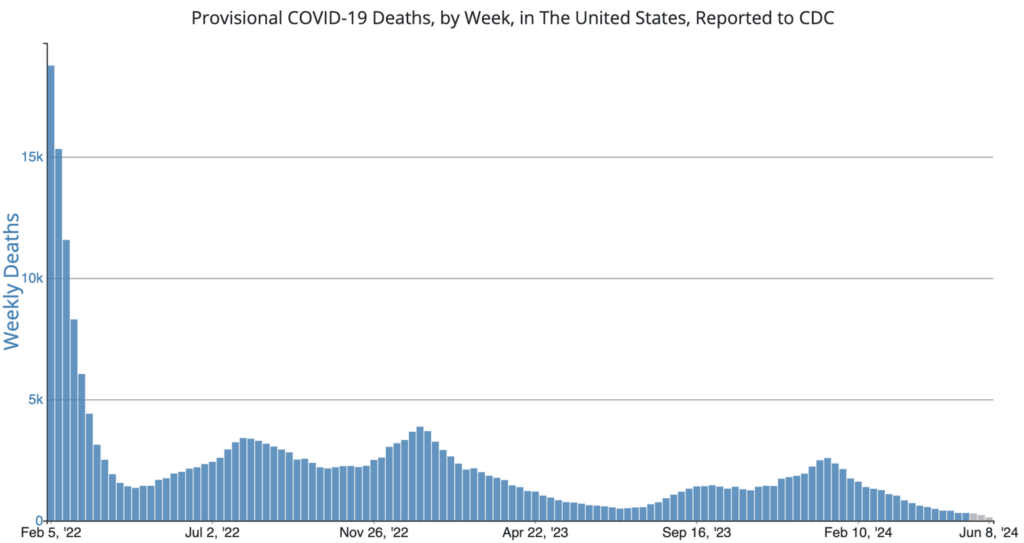
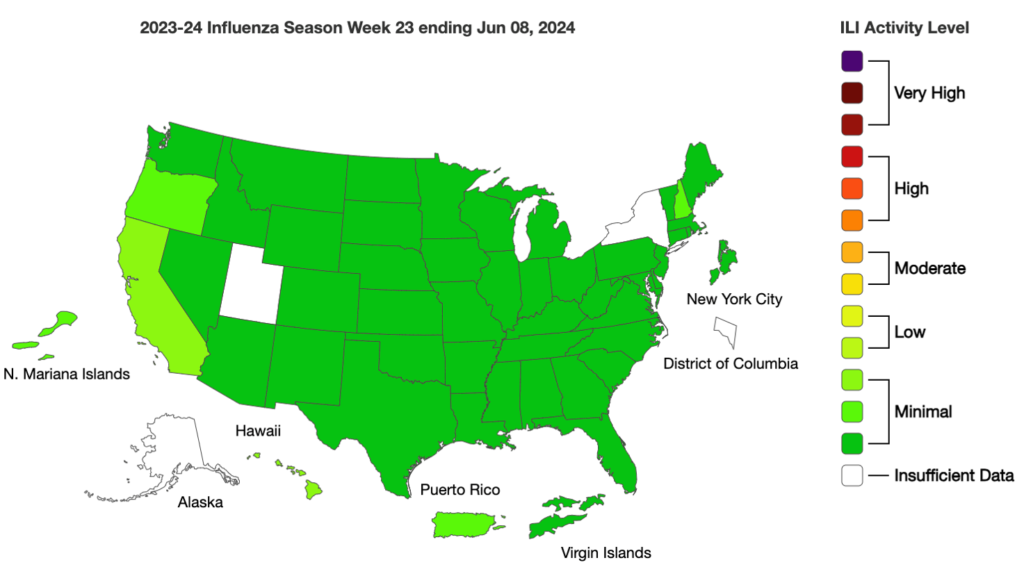
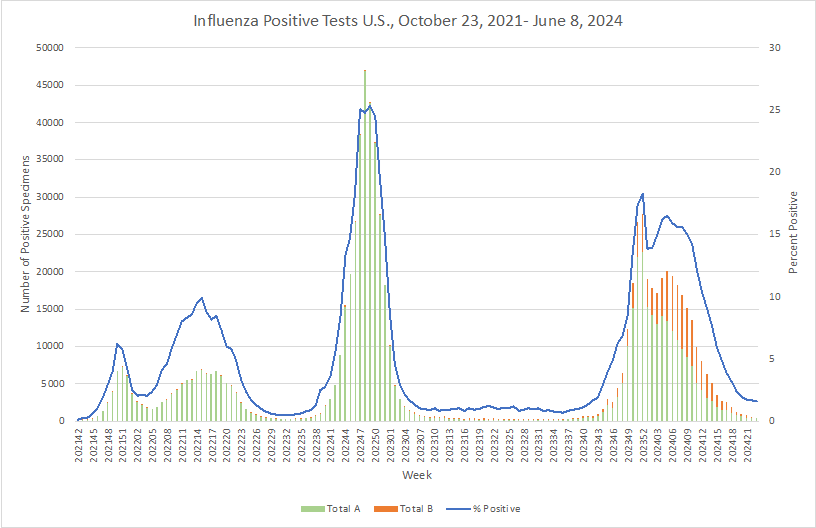
Influenza:
- On June 10, 2024, Moderna announced that its Phase 3 trial of mRNA-1083, an investigational combination vaccine against influenza and COVID-19, has met its primary endpoints, eliciting a higher immune response than the licensed comparator vaccines for Flu and COVID-19 used in the trial as separate vaccines. Engagement with regulators is the next step. The combination vaccine could be available in the Fall of 2025.
- The US CDC reports the emergence and intercontinental spread of pH1N1 viruses displaying reduced susceptibility to oseltamivir (e.g., Tamiflu) resulting from acquisition of 2 mutations. These findings stress the need for continued surveillance for mutated strains.
- Roche Diagnostics is releasing a PCR test to determine if a patient has COVID-19, Influenza A, Influenza Flu B or RSV.
- Avian flu has been detected in cattle at two dairy herds in northwest Iowa, raising the total number of affected herds in the state to five, according to the Iowa Department of Agriculture and Land Stewardship. The virus, which significantly reduces milk production but is generally non-lethal to cows, was found in a 3,000-cow herd in Plymouth County and a 1,000-cow herd in Sioux County.
- Last week the second death from mpox was reported in South Africa, less than 24 hours from the first. The deaths were recorded from two men, aged 37 and 38.
- Though the US FDA last week recommended that vaccine companies switch to the JN.1 variant for updated COVID shots for the fall, since then they have urged companies to focus on the KP.2 lineage if possible, based on recent shifts and a national rise in cases.