With cases of H5N1 in domestic and wild cats in California, Colorado, Oregon, and Washington State found to be associated with eating contaminated food products, the FDA is now requiring that cat and dog food manufacturers subject to the Preventive Controls for Animal Foods rule consider H5N1 to be a known or reasonably foreseeable hazard. This means that manufacturers using uncooked or unpasteurized materials derived from poultry or cattle are to reanalyze their food safety plans to include Highly Pathogenic Avian Influenza virus (specifically H5N1).
The requirement is of most concern in cat food, as they can become seriously ill or die from an H5N1 infection. Although dogs can also contract H5N1, they usually have milder symptoms and low mortality; additionally, H5N1 has not been detected in dogs in the U.S., though there have been fatal cases in other countries.
To help minimize or prevent transmission through animal foods, FDA is also recommending that manufacturers seek ingredients from flocks or herds that are healthy, and implement processing steps, such as heat treatment, that are capable of inactivating viruses, and/or implement a supply-chain-applied control to provide assurance that ingredients do not come from H5N1-infected animals.
Because the virus can survive on surfaces for extended times, TAG recommends that facilities provide awareness training about HPAI, including its risks and how it can be managed. Additionally, not only should establishments follow proper sanitation (e.g., cleaning followed by use of sanitizer) and standard GMPs, but also minimize the risk of introducing HPAI into the facility through additional hygienic controls, particularly for workers who may have direct or indirect contact with poultry. For custom recommendations related to your facility and processes, or assistance with your public health plan, contact TAG.
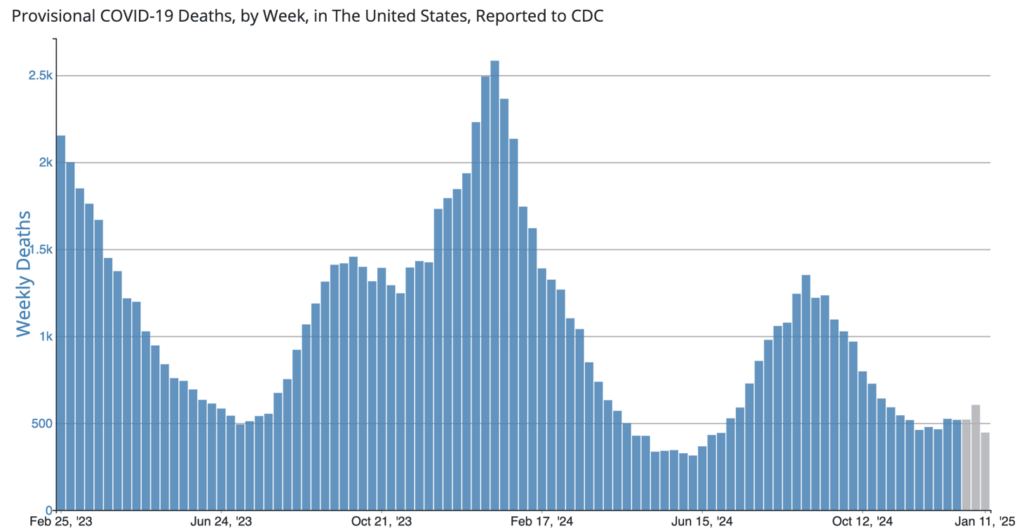
COVID Risk Matrix:
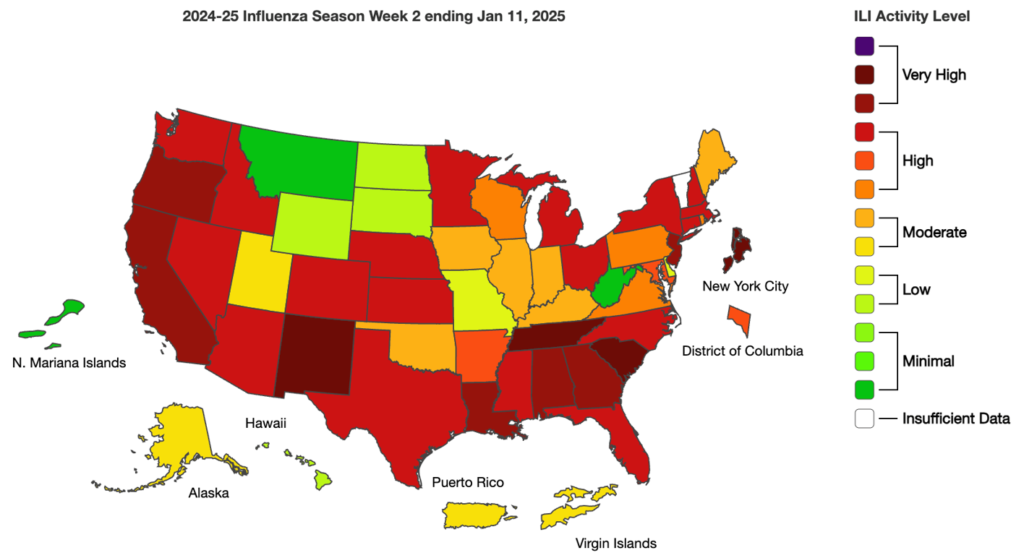
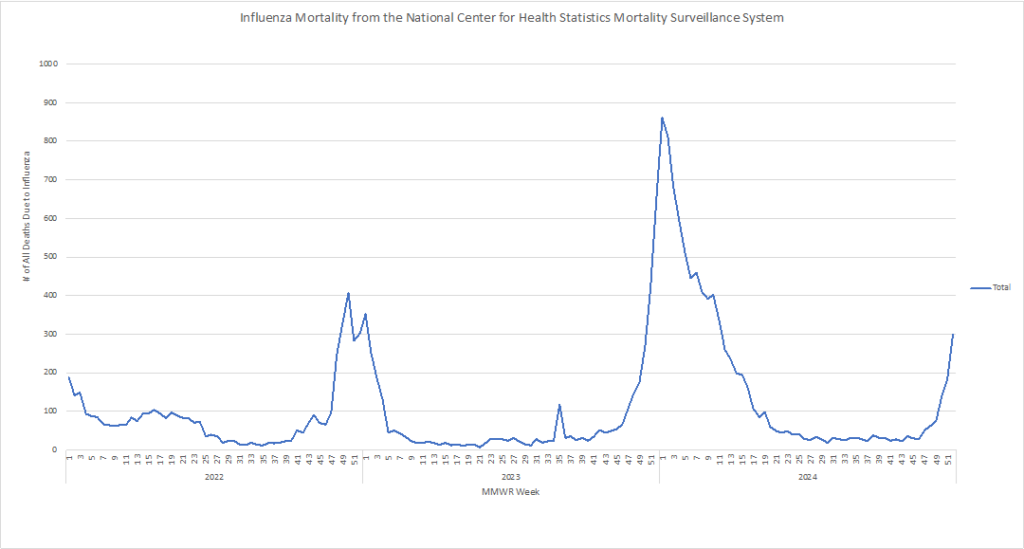
Influenza:
Infectious Disease News:
- WHO is investigating a possible outbreak of Marburg virus in Tanzania, although all samples tested to date have been negative. 9 suspect cases have been reported, and 8 deaths have been reported. Follow-up surveillance of ~300 contacts, including healthcare workers and further virus characterization is underway. Marburg virus disease is a rare but severe hemorrhagic fever that can lead to serious illness and death. There is no treatment or vaccine. It is in the same family as Ebola, is transmitted from fruit bats, and spreads among people through direct contact with bodily fluids.
- Meningitis cases have resurged in northern Togo, which is part of the so-called African meningitis “belt” where large-scale epidemics occur every 5–12 years. The current outbreak is believed to be caused by Strep. pneumoniae. This part of Africa typically experiences dry conditions, leading to excessive dust that can facilitate bacterial spread. Enhanced surveillance and appropriate vaccinations are recommended.
- The FDA released a strategy aimed at preventing the contamination of fresh and frozen berries with enteric viruses including Hepatitis and norovirus. Elements include compliance with FDA requirements; use of root cause analysis; continued research on the viability, persistence, detection, and mitigation of viruses in berries and growing environments; and incentives for immunization programs.
- A feeding study in monkeys suggests that drinking raw milk contaminated with highly pathogenic H5N1 avian flu is a risk for infection but may lead to less severe illness than respiratory tract exposure to the virus.
- The FDA has determined that it is necessary for manufacturers of cat and dog foods, who are covered by the Preventive Controls for Animal Food rule and using uncooked or unpasteurized materials derived from poultry or cattle, to reanalyze their food safety plans to include Highly Pathogenic Avian Influenza virus (specifically H5N1) as a known or reasonably foreseeable hazard.
- TAG has created an HMPV Fact Sheet, which can be accessed for free on our website. Find it here.





