It’s that time of year again. Seasonal respiratory viruses are continuing to increase, so if you haven’t yet gotten your flu and COVID vaccines, you can expect to get constant reminders from your healthcare provider, pharmacies, and TV ads to do so. While the options this year are similar to previous years, with vaccines updated to fight the current strains, next year’s options are likely to expand to an at-home nasal spray option for the flu.
On September 20, the FDA approved FluMist for self- or caregiver-administration for the prevention of influenza disease caused by influenza virus subtypes A and B in individuals 2 through 49. First approved for ages 5 to 49 in 2003, then for ages 2 to 5 in 2007, the new self-administration vaccine enables at-home use of the nasal-spray vaccine. It is the first influenza prevention vaccine that does not need to be administered by a health care provider. However, a prescription is still required for the FluMist vaccine which contains a weakened form of live influenza virus strains and is sprayed in the nose.
Although the self-administered use has been approved by FDA, FluMist expects the vaccine, which is currently available at doctors’ offices for ages 2 to 49, to be available for the 2025-26 flu season through home delivery. Those interested would place an online order, complete a medical questionnaire, provide insurance information, select a delivery date, and provide shipping payment. A pharmacist would then review the information and, if approved, the vaccine would be delivered to the home. At this time, people can register to be informed of when FluMist will be available in their area.
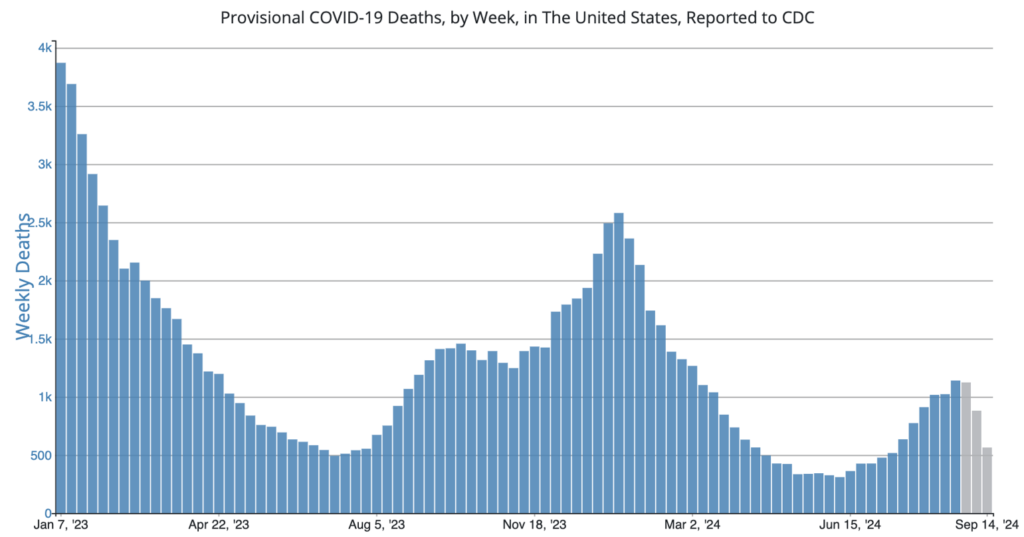
COVID Risk Matrix:
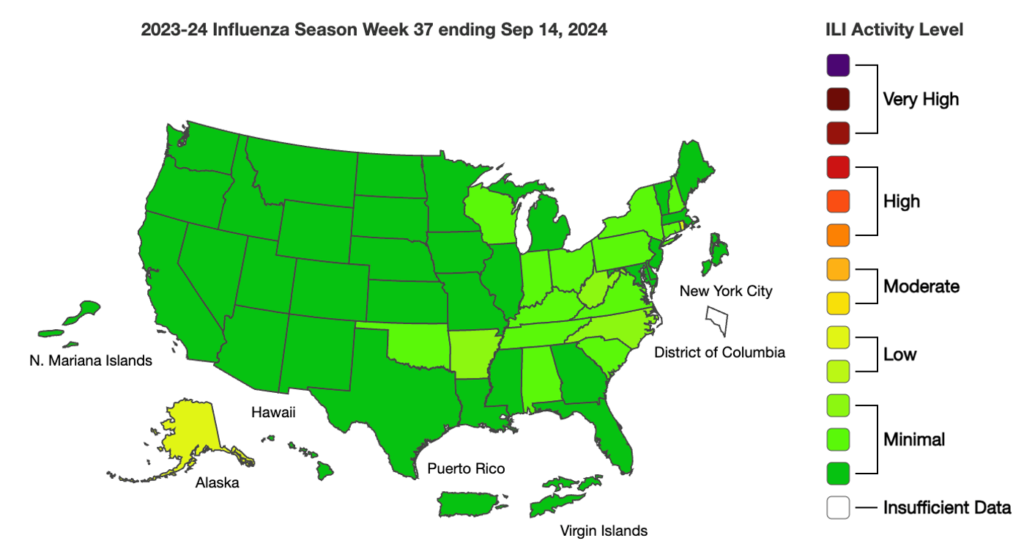
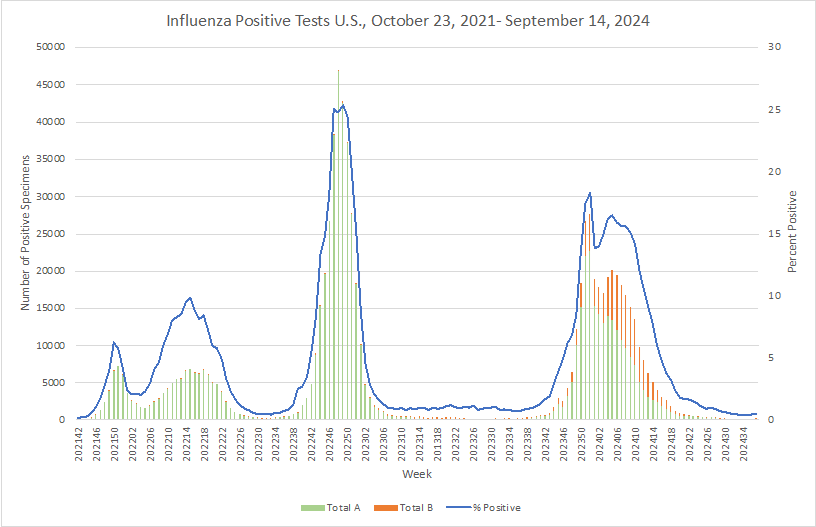
Influenza:
- Health Canada has approved an updated version of Moderna’s COVID-19 vaccine, which targets recent variants of the virus. The decision means that millions of new mRNA shots will start arriving in Canada within days, with distribution decisions left to provincial and territorial governments. While Pfizer-BioNTech’s updated vaccine is still pending approval, both revamped vaccines aim to counter evolving COVID variants that threaten existing immunity. Despite a recent rise in cases, hybrid immunity continues to protect most Canadians from severe illness, especially among high-risk populations.
- The U.S. FDA has approved FluMist for self- or caregiver-administration, making it the first influenza vaccine that doesn’t require a healthcare provider for administration. Approved for individuals aged 2 to 49, FluMist is a nasal spray containing weakened live influenza virus strains. This approval provides a more convenient option for flu prevention, aiming to increase accessibility and flexibility in vaccination. FluMist will still require a prescription, and it will be available through a third-party online pharmacy next year with an eligibility assessment.
- Cases of meningitis have doubled in England in just one year. The number of cases is close to pre-pandemic levels. Vaccination is urged.
- Reports of increasing whooping cough cases continue. Examples include Alaska, with 286 cases; Ontario with more than 1,000 cases; and Wisconsin, with cases 10 times higher than last year.
- Rwanda has begun vaccinating people in high-risk areas against mpox – the first country in Africa to do so. In the first two days of efforts about 500 people were vaccinated. In other mpox news, India confirmed their first case of the new strain (clade 1b) just recently.





