With the US declaring an end to the COVID-19 public health emergency (PHE) as of May 11, 2023, CDC has updated its guidance to incorporate some changes:
- Neither at-home coronavirus test kits nor those performed by clinicians and analyzed by commercial labs will continue to be free. Some insurance carriers may still cover the vaccines, but they are no longer required to do so.
- Access to COVID-19 vaccines will generally not be affected, for now – but there may be some changes in the vaccines that can be obtained at pharmacies, especially childhood vaccines. However, COVID vaccines will remain free for those with and without insurance at least through December 2024.
- Treatments, such as Paxlovid, will also remain free while they last. After that, the price will be determined by the insurance carrier.
- Labs will no longer be required to report COVID test results to CDC, so updating guidance in response to illness statistics will be more challenging. Some data will continue to be available (as outlined by CDC).
- Proof of vaccination – The US has cancelled vaccination requirements for international travel. COVID is no longer a global public health emergency according to the World Health Organization (WHO).
Although the public health emergency aspect of COVID is declared over, COVID has not disappeared. You can still get and spread COVID, so it’s wise to continue to remind employees to stay home when they are ill.
The end of the PHE also provides a good opportunity to review your COVID protections to determine what worked, and what didn’t, while it is still fresh. If you have a plan, use this review to make revisions to help ensure you are more prepared for other emerging infectious diseases. If you did not have a plan, reviewing the protections you implemented can help you create one to put you a step ahead as well.
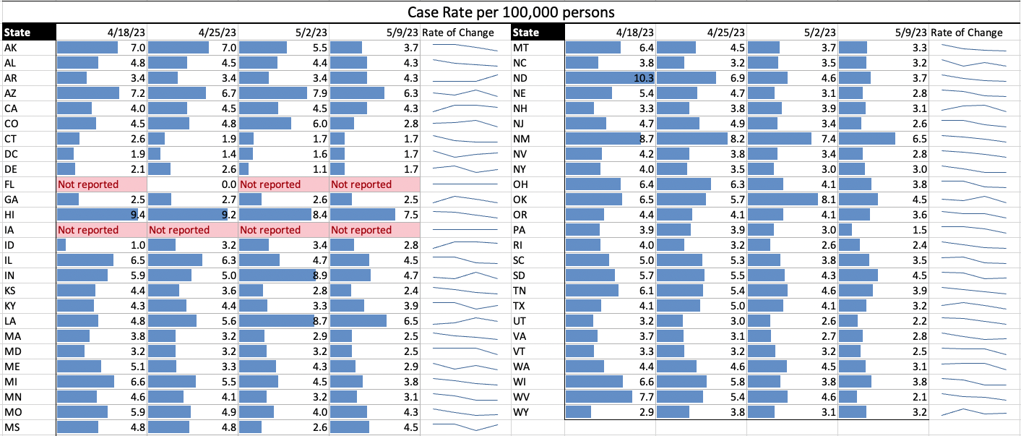
COVID Risk Matrix:
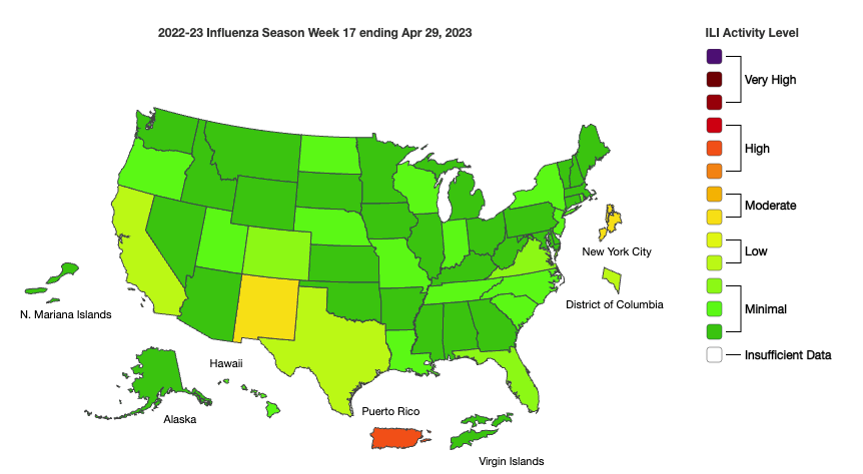
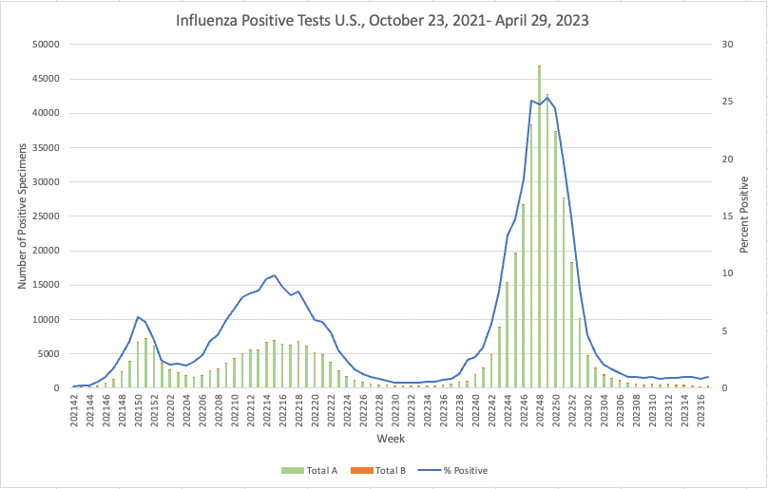
Influenza:
Infectious Disease News:
- COVID. As declared by the CDC, the end of the COVID Public Health Emergency in the US is on May 11.
- Measles. In South Africa, in the past weeks (weeks 15 up until week 16, 4/18/23) there have been 20 laboratory confirmed measles cases detected across all regions of the country. A majority of the cases were from Limpopo in the North.
- RSV. FDA has approved the nation’s first RSV vaccine for people 60 and older. The vaccine is made by GlacoSmithKline and sold under the brand name Arexvy. In a study, the vaccine reduced the risk of RSV-related lower respiratory track disease (LRTD) by 82.6% and lowered the risk of severe LRTD by 94.1%.
- Pancreatic Cancer. A new study from Nature Medicine suggests that an AI population screening may now be able to predict whether someone will get pancreatic cancer up to three years in advance. Population screening is when medical professionals perform genetic testing or similar methods to look at the prevalence of a specific trait that is found among a group of people.





