Since the pandemic, mass gatherings have carried concerns about the potential for the spread of communicable disease. And the 2024 Olympic and Paralympic Games in Paris are no exception. Because of that, public health officials have been heavily involved in ensuring health and safety of participants and spectators.
With the Games involving about 15,000 athletes, up to 50,000 volunteers, and a projected 15 million visitors to attend the Olympics and/or the Paralympics, and major sporting events requiring particular vigilance, Santé publique France was asked to create a public health risk map. It was determined that particular attention would be paid to foodborne infections, certain infectious diseases and heat-related risks in the event of a heatwave.
From the findings of the risk map, and its own ongoing monitoring of the Games, the European CDC (ECDC) determined the probability of communicable disease transmission to be low if general preventive measures were applied. Thus far, that has seemed to be true with little more than COVID-19 cases reported among athletes from the Australian Polo Women’s Team, the US Swimming Team, and the Great Britain Swimming Team and some illnesses among teams that swam in the Seine River in which the bacterial levels have fluctuated daily.
The preventive measures being recommended for the athletes, volunteers, and visitors include such practices as being fully vaccinated according to the national immunization schedules, following hand and food hygiene and respiratory etiquette, self isolating with flu-like symptoms until they resolve, wearing a mask in crowded settings, seeking prompt testing and medical advice as needed, and practicing safe sex, as per guidance provided by the French authorities.
This combination of preventive measures, along with those of the public health authorities has had an effect, with the latest ECDC Communicable Disease Threats Report weekly bulletin reporting no major public health events related to communicable diseases detected related to the Olympic Games. Monitoring via epidemic intelligence activities will continue through September 13, 2024.
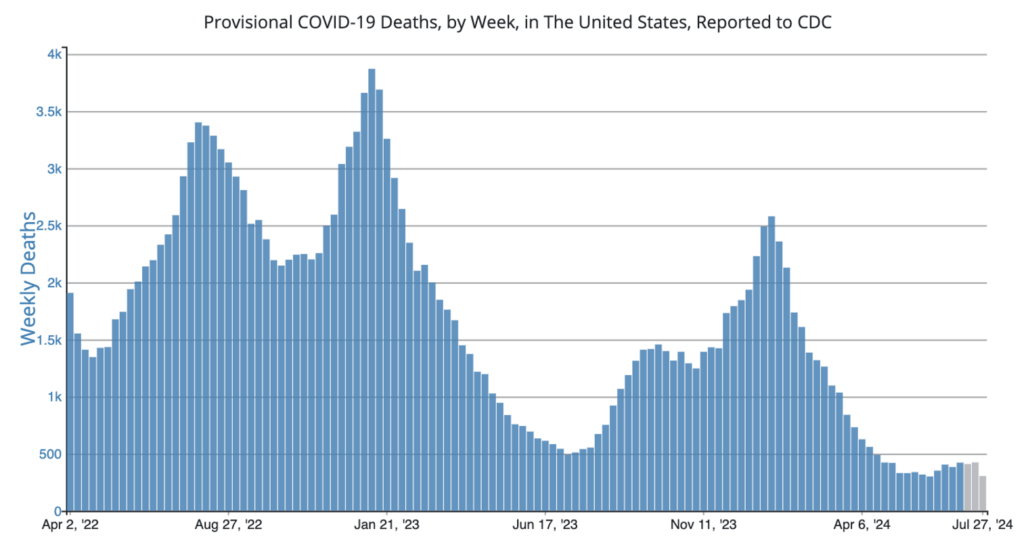
COVID Risk Matrix:
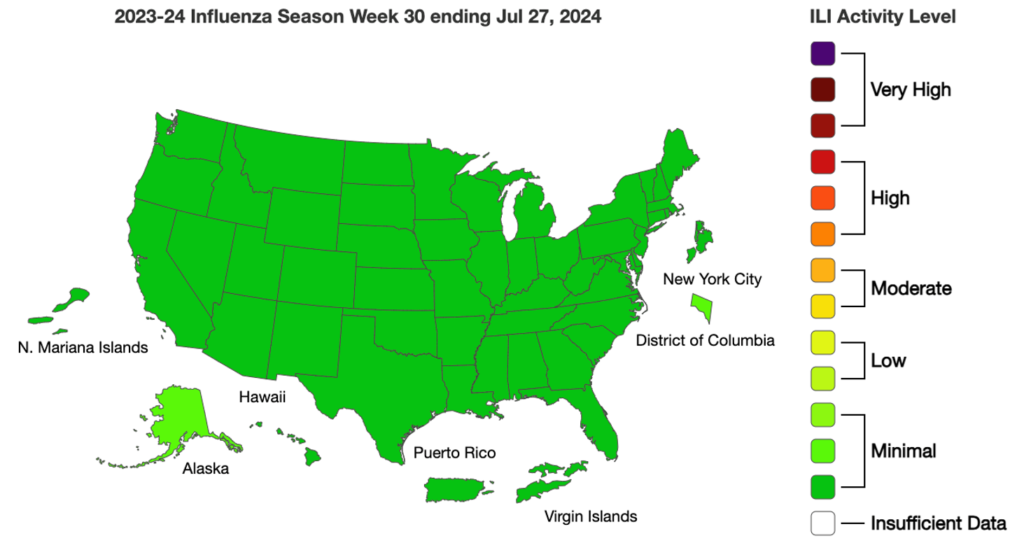
Influenza:
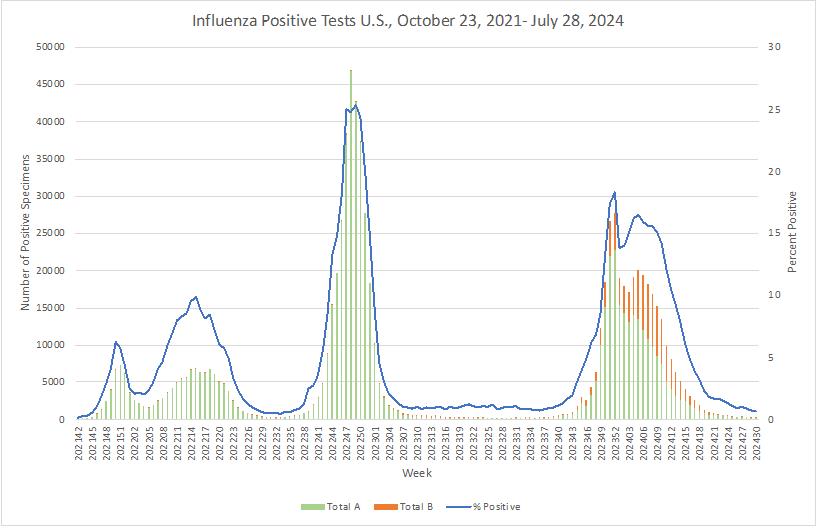
- The best time to get a flu vaccine is September or October, with a few exceptions, the CDC says. Pregnant women in their third trimester are encouraged to get vaccinated in July or August to protect their unborn child from the flu during the first few months of life when they are too young to get vaccinated. Consult medical professionals for further advice.
- Stem cell therapy has demonstrated success in treating severe COVID-19 cases and several clinical trials have revealed it improves 28-day survival rates, reduces mortality, and accelerates recovery. Stem cells are found in many tissues in the body, including bone marrow, fat tissue, and umbilical cord.
- Two people have died from Legionnaires’ disease in Melbourne, Australia with at least 77 cases identified and dozens of people hospitalized. No source has yet been identified though testing of cooling towers continues.
- 203 measles cases have been reported across 25 states, New York City and the District of Columbia as of Aug 1, 2024. In all of 2023, there were just 58 cases recorded. Among the cases, 40% have occurred in children under the age of 5, while 32% were among individuals 20 and older. Approximately 95% of cases have involved individuals who were either unvaccinated, had an unknown vaccination status or had received only one dose of the measles, mumps and rubella vaccine.
- WHO is considering declaring the mpox outbreak in Africa a public health emergency of international concern (PHEIC). The outbreak, caused by the more deadly clade 1b of the virus, originated in the Democratic Republic of the Congo (DRC) and has spread to other African countries, including South Africa and Uganda. The DRC reports 13,791 cases and 450 deaths, while South Africa has 22 cases with 3 deaths. The WHO previously declared a PHEIC for the 2022 mpox outbreak, which was controlled with vaccines not widely available in Africa. The DRC has approved two mpox vaccines, with 50,000 doses donated by the U.S., but they have yet to be utilized.
- Indian vaccine company Biological E announced that WHO has prequalified its novel oral poliovirus type 2 (nOPV2) vaccine. This next-generation vaccine is designed to combat circulating vaccine-derived poliovirus type 2 (cVDPV2) outbreaks with improved genetic stability, reducing the risk of seeding new outbreaks in low-immunity areas. In collaboration with Indonesia-based PT Bio Farma and supported by the Bill and Melinda Gates Foundation, Biological E can produce over 500 million doses annually. Indian regulators have approved the vaccine for export, and its real-world deployment has shown promise in reducing cVDPV2 outbreaks.





