When an outbreak has been identified, the next step is to stop the outbreak by first tracing and then cutting off routes of transmission. Such tracking can be done through spatiotemporal clustering and contact tracing, but as the report states, this is effective but “extremely labor-intensive and can involve large-scale chart reviews, random environmental sampling, and in-depth interviews.” Tracking also can be done through tying cases together through genetic similarities of whole genome sequence (WGS) surveillance. However, this method cannot identify sources of infection and may fail to identify complex transmission patterns needed to direct interventions.
By taking WGS a step further, adding a machine-learning (AI) layer, University of Pittsburgh researchers created an outbreak source identification system, which automatically mines patients’ electronic medical records for data related to an outbreak. It was found that running a real-time AI algorithm to detect what was being missed by traditional methods resulted in early disease recognition, infection prevention, a substantial decrease in potential illness, and cost savings.
Just as the clustering/contact tracing and WGS surveillance alone showed limitations in the hospital settings, so, too, do they hold limitations in foodborne outbreak investigations. However, with the study showing such positive results in a WGS/AI combination, a similar approach could be used for comparing WGS isolates and epidemiologic exposure data in foodborne illness outbreaks. FDA could query a broader portion of the supply chain and feed product distribution data in the algorithm to rapidly trace common lot codes through the supply chain.
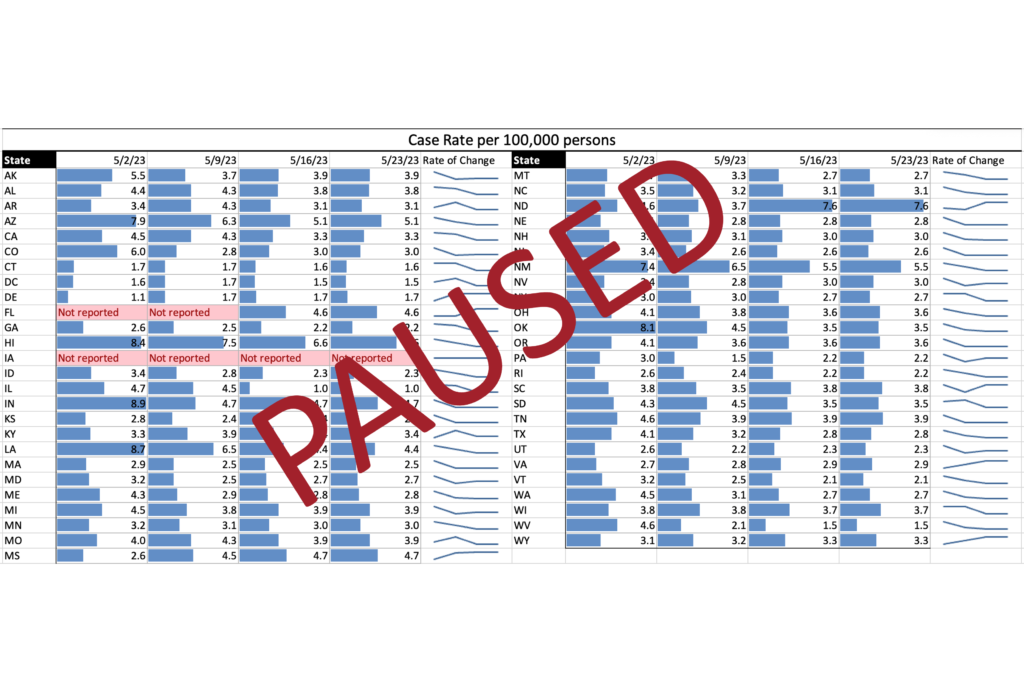
COVID Risk Matrix:
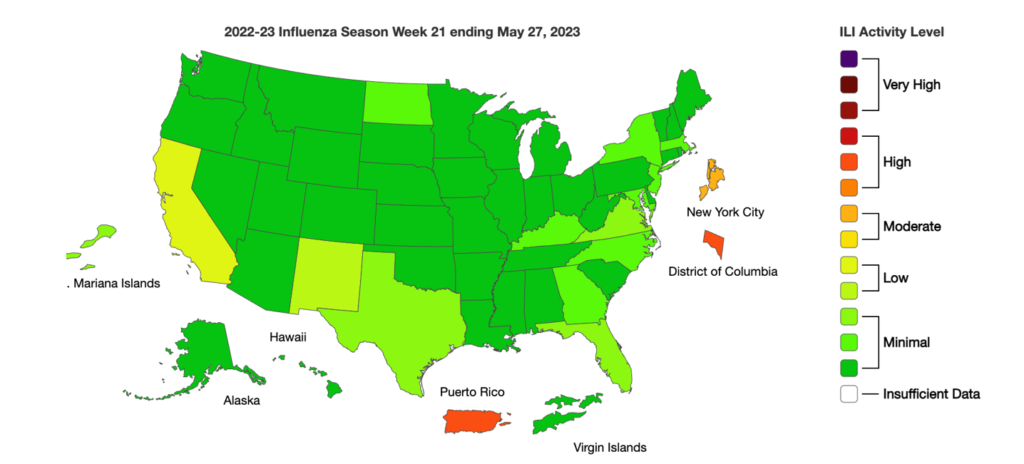
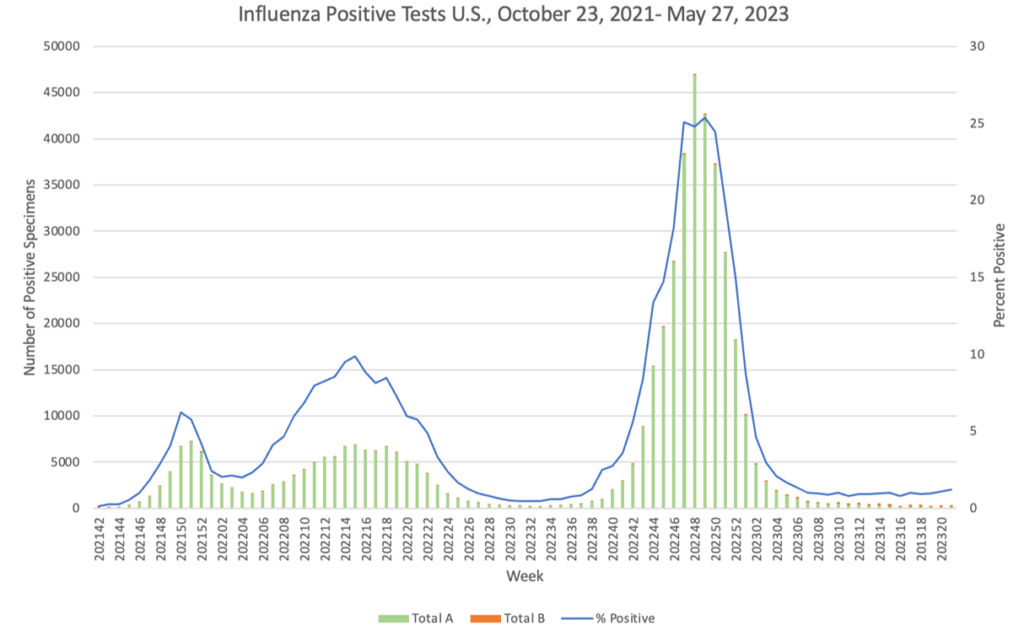
Influenza:
- FAO has launched a comprehensive and practical toolbox designed to help all those operating in the food sector adhere to international food hygiene standards. The toolbox is based on the Codex Alimentarius which aims to protect consumer health and promote fair practices in food trade.
- The FDA revoked the emergency use authorization (EUA) of the Janssen COVID-19 vaccine. The company requested voluntary withdrawal of the EUA for the vaccine. They do not plan to update the vaccine to different strain composition to address emerging variants.
- South Africa has reported an increase in flu cases among school children and workers. Australia and Chile have reported rises in flu cases this past week as well.
- Throughout 2022 in the US, cases of TB decreased through the pandemic, likely due to missed diagnoses or changes in travel practices. In 2022, there were more cases among non-US-born persons newly arrived in the US; higher TB incidence among Hispanic, American Indian, or Alaska Native and non-Hispanic, Native Hawaiian, or other Pacific Islander persons and persons aged ≤4 and 15–24 years; and slightly lower incidence among persons aged ≥65 years. TB incidence levels seem to be returning to pre-pandemic levels.
- A Tacoma woman diagnosed with TB who refused treatment for more than a year was arrested Thursday by Pierce County deputies to be isolated and treated in jail, to protect the public.
- In Israel, at least 215 cases of whooping cough have been reported to the Health Ministry since January, accounting for a 12-fold increase over the same period in 2022, when there were only 17 cases. The majority are in Jerusalem and are likely attributed to lower vaccination rates since COVID.
- In the ongoing outbreak of meningitis associated with surgical procedures in those who traveled to clinics in Matamoros, across the border from Brownsville, Texas, the CDC confirmed the presence of the fungus Fusarium solani in two US cases and updated the number of fatalities from two to three.
- Canadian surveillance showed that among 88 hospitals from January 2017 through December 2021, MRSA bloodstream infections rose 35%. The increase was driven by community-associated cases, which climbed by 80% over the study period while healthcare-associated cases remained stable.
- The FDA has approved another RSV vaccine, this one made by Pfizer. In early May, the FDA approved GlaxoSmithKline’s RSV vaccine for individuals 60 years and older, the same age group applies for the Pfizer vaccine. Pfizer’s vaccine contains antigens against RSV subgroups A and B.
- Although human metapneumovirus (HMPV) hit some high levels in the spring, rates have decreased significantly. More information on the virus is available on TAG’s Infectious Disease Fact Sheets web page.





